This technical guide explains how to convert milligrams to milliliters accurately for medical formulations safely.
Describes algorithms, density handling, concentration units, and validation methods for free online calculator tools today.
Mass (mg) to Volume (mL) Conversion — Precise Online Calculator
Fundamental concepts for converting mg to mL
Converting milligrams (mg) — a unit of mass — to milliliters (mL) — a unit of volume — requires an explicit link between mass and volume. That link is provided either by concentration (typically expressed as mg/mL, % w/v, or ratio strength) or by density (g/mL or mg/mL) for pure substances or non-aqueous liquids. Any online calculator claiming to convert mg to mL must therefore solicit the correct contextual parameter: concentration or density.
Mass, volume, concentration, and density — definitions
- Mass (mg, g): amount of matter. 1 g = 1000 mg.
- Volume (mL, L): space occupied. 1 L = 1000 mL.
- Concentration (mg/mL): mass of analyte per unit volume of solution. Direct conversion: mL = mg ÷ (mg/mL).
- Density (g/mL): mass per unit volume of a substance (pure liquid). To convert mg of a pure liquid to mL: convert mg to g then divide by density.
Core formulas and variable explanations
All formulas below use simple arithmetic and explicit unit conversions. Each formula is followed by variable explanations and typical numerical values.
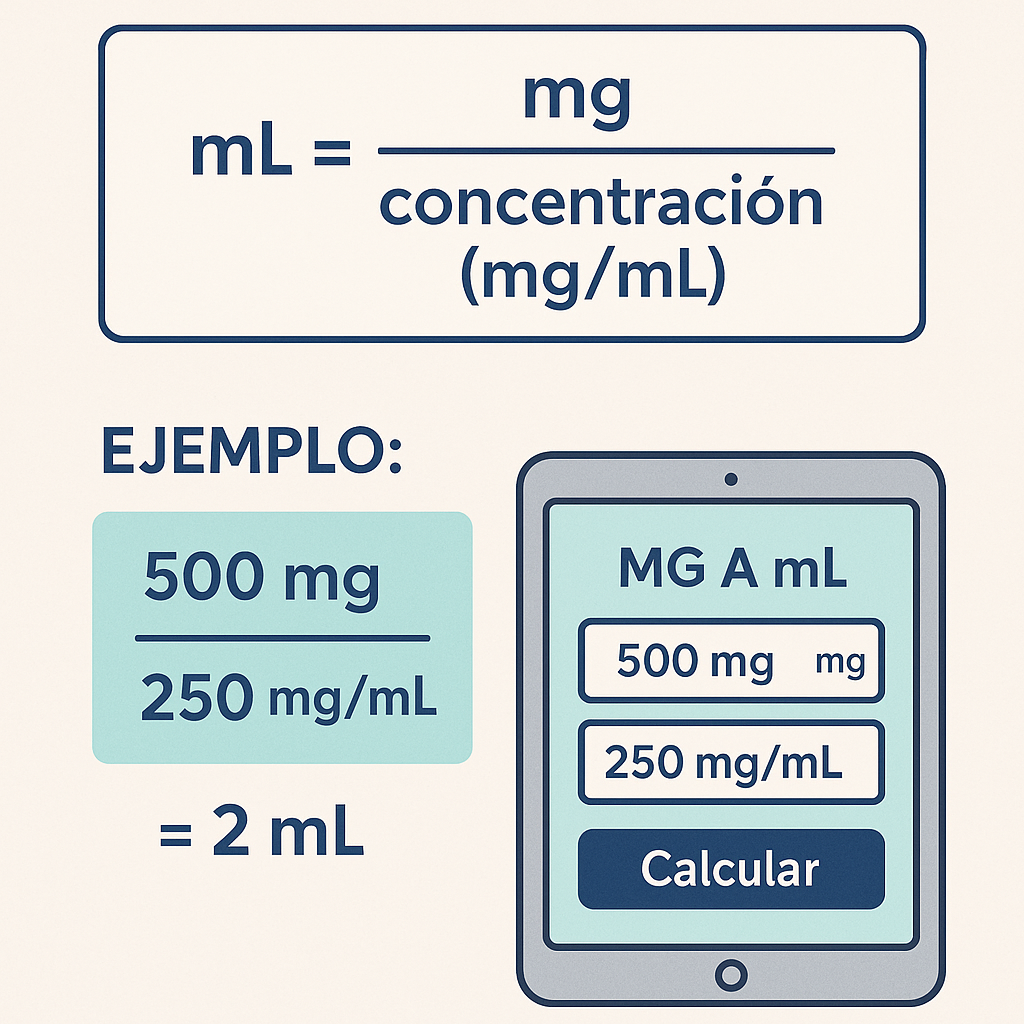
Formula for solutions with known concentration (most common clinical use):
mL = mg ÷ C
Where:
- mL = required volume in milliliters.
- mg = prescribed or measured mass in milligrams.
- C = concentration expressed in mg/mL.
Typical values for C: 1 mg/mL, 2 mg/mL, 5 mg/mL, 10 mg/mL, 100 mg/mL (depends on preparation).
Formula for % w/v to mg/mL (common in pharmacy):
mg_per_mL = (% w/v) × 10
- 1% w/v = 1 g/100 mL = 1000 mg/100 mL = 10 mg/mL.
- Therefore, to convert mg dose to volume: mL = mg ÷ ( (% w/v × 10 ) ).
Formula for ratio strength (e.g., 1:1000):
C = (1 g ÷ denominator) × 1000 mg/g ÷ 1 mL leading to simpler expression: C = 1000 ÷ denominator (mg/mL)
Example: 1:1000 → C = 1000 ÷ 1000 = 1 mg/mL.
Formula for converting mass of pure liquid to volume using density:
mL = (mg ÷ 1000) ÷ ρ
Where:
- mg ÷ 1000 converts milligrams to grams.
- ρ (rho) is density in grams per milliliter (g/mL).
- Thus: volume (mL) = mass (g) / density (g/mL).
Typical densities: water = 1.000 g/mL; ethanol ≈ 0.789 g/mL (20 °C); glycerol ≈ 1.26 g/mL.
Edge cases and unit-sanity checks
- Zero or near-zero concentrations: calculators must prevent division by zero and alert for unrealistic concentrations.
- Unit mismatch: ensure concentration units match the mass units (mg vs g). If concentration is g/mL, convert mass to g first.
- Temperature-dependent densities: include temperature field when dealing with volatile solvents (ethanol, isopropanol) if high precision required.
- Significant figures: preserve clinical significance — if a dose is specified to two significant figures, reflect that in volume rounding rules.
Design logic and algorithm for an accurate online calculator
An accurate free online mg → mL calculator follows a deterministic decision tree:
- Input: requested mass in mg.
- Ask: Is the substance a component of a solution (concentration known) or a pure liquid (density known)?
- If solution: allow selection of concentration unit (mg/mL, % w/v, ratio). Convert selected unit to mg/mL.
- If pure liquid: accept density (g/mL) and temperature; convert mg → g; compute volume using density.
- Validate inputs (non-zero, non-negative, plausible ranges).
- Compute mL using formulas above and present stepwise calculation and rounding options.
- Provide warnings for extreme results (e.g., volumes less than 0.01 mL) and recommended pipette sizes or administration routes.
Common concentrations and equivalences
| Label / Form | Expression | Equivalent mg/mL | Notes |
|---|---|---|---|
| 0.1% w/v | 0.1 g / 100 mL | 1 mg/mL | Low concentration ophthalmic or topical examples. |
| 0.5% w/v | 0.5 g / 100 mL | 5 mg/mL | Frequently used for dilute anesthetic preparations. |
| 1% w/v | 1 g / 100 mL | 10 mg/mL | Standard reference point: 1% = 10 mg/mL. |
| 2% w/v | 2 g / 100 mL | 20 mg/mL | Common in local anesthetic formulations. |
| 5% w/v | 5 g / 100 mL | 50 mg/mL | Concentrated solutions — ensure appropriate dilution before administration. |
| 10% w/v | 10 g / 100 mL | 100 mg/mL | Used in compounding and some IV preparations. |
| 1:10 | 1 g / 10 mL | 100 mg/mL | Ratio representation common in older pharmacopoeial literature. |
| 1:100 | 1 g / 100 mL | 10 mg/mL | Equivalent to 1% w/v. |
| 1:1000 | 1 g / 1000 mL | 1 mg/mL | Epinephrine 1:1000 is 1 mg/mL. |
Common densities for liquids relevant to mg-to-mL conversion
| Substance | Density (g/mL) | Temperature reference | Source / Typical use |
|---|---|---|---|
| Water | 1.000 | 4–25 °C (standard) | Universal reference; most aqueous pharmaceutical vehicles. |
| Sodium chloride solution (0.9% w/v) | ≈1.005 | 20 °C | Physiological saline for IV infusion; density slightly > water. |
| Ethanol | 0.789 | 20 °C | Sterilants, preservatives, tinctures. |
| Glycerol (glycerin) | 1.26 | 20 °C | Viscous excipient used in topical preparations. |
| Benzyl alcohol | 1.04 | 20 °C | Preservative in some parenteral solutions. |
| Isopropyl alcohol (70%) | ≈0.88 | 20 °C | Antiseptic solutions; mixture density depends on concentration. |
Implementation details for a high-precision free online calculator
To achieve clinical-grade precision and provide a useful tool for pharmacists and clinicians, implement the following functionality:
- Input validation: numeric types, positive values, controlled ranges.
- Unit picker: allow mg, g, μg for mass; mg/mL, g/mL, % w/v, ratio strength for concentration; g/mL for density.
- Auto-conversion: behind the scenes convert all inputs to mg and mg/mL or g and g/mL for density calculation.
- Explain-step output: show each conversion step to aid clinical verification.
- Rounding options: user-selectable significant figures or fixed decimal places (e.g., two decimal places for volumes >1 mL, three or more for micropipetting).
- Warnings & tooltips: tiny volumes (<0.05 mL) prompt minimum handling warnings; large volumes prompt dilution advice.
- Audit trail: store calculation parameters, timestamp, and user ID for institutional validation.
Precision sources and uncertainty budgeting
High precision demands quantification of error sources:
- Concentration uncertainty: labeled concentrations often have ±% tolerances per batch or pharmacopeial standards.
- Density variability: temperature and purity changes affect density; use NIST or manufacturer data for high precision.
- Device dispensing error: syringe and pipette errors contribute to final concentration; include recommended tolerances.
- Rounding error: truncated display can hide small but clinically relevant deviations; offer extended precision on demand.
Examples with step-by-step development and final solutions
Example 1 — solution concentration case (typical clinical dose)
Scenario: A clinician orders 250 mg of Drug X. The available vial concentration: 5 mg/mL. Determine the volume to administer.
Step 1 — Identify formula: mL = mg ÷ C.
Step 2 — Substitute values: mg = 250 mg; C = 5 mg/mL.
Step 3 — Compute: mL = 250 ÷ 5 = 50 mL.
Step 4 — Rounding and checks: 50 mL is a practical volume; verify vial volume availability and infusion rate if required.
Final solution: 250 mg of Drug X requires 50.0 mL of a 5 mg/mL solution.
Example 2 — percent w/v conversion
Scenario: Pharmacy must prepare 5 mg of Compound Y from a 2% w/v stock solution. Determine volume required.
Step 1 — Convert 2% w/v to mg/mL: mg_per_mL = 2 × 10 = 20 mg/mL.
Step 2 — Apply basic formula: mL = mg ÷ C = 5 ÷ 20 = 0.25 mL.
Step 3 — Handling and accuracy: 0.25 mL is 250 µL; recommend a calibrated syringe or micropipette for accuracy.
Final solution: 5 mg requires 0.25 mL of the 2% w/v stock, dispensed with an appropriate micropipette.
Example 3 — density-based conversion for pure liquid
Scenario: A laboratory needs to measure 50 mg of ethanol in liquid form for a formulation. Ethanol density at 20 °C = 0.789 g/mL. Compute the volume.
Step 1 — Convert mg to g: 50 mg ÷ 1000 = 0.050 g.
Step 2 — Use density formula: mL = mass (g) ÷ density (g/mL) = 0.050 ÷ 0.789.
Step 3 — Compute numeric result: 0.050 ÷ 0.789 ≈ 0.06335 mL.
Step 4 — Convert to µL for dispensing: 0.06335 mL × 1000 = 63.35 µL.
Final solution: 50 mg ethanol corresponds to approximately 0.0634 mL (≈63.35 µL). Use precision micropipette; account for evaporative losses and temperature effects on density.
Example 4 — ratio strength in emergency medication
Scenario: Administer 0.3 mg of epinephrine from a 1:1000 ampoule used in emergency care. Compute volume.
Step 1 — Resolve ratio: 1:1000 → C = 1000 ÷ 1000 = 1 mg/mL.
Step 2 — Compute volume: mL = mg ÷ C = 0.3 ÷ 1 = 0.3 mL.
Step 3 — Clinical notes: 0.3 mL is easily drawn from a 1 mL syringe; verify concentration labeling before administration.
Final solution: Draw 0.3 mL of epinephrine 1:1000 to deliver 0.3 mg.
Recommended validations, references, and regulatory context
For clinical and compounding use, calculators should conform to pharmacopeial and regulatory guidance. Relevant authoritative sources include:
- United States Pharmacopeia (USP) — standards for compounding and concentration labeling (see USP chapters USP General Chapters and chapters 795/797/800 as applicable).
- European Medicines Agency (EMA) — guidance for medicinal product quality and labeling: EMA.
- U.S. Food and Drug Administration (FDA) — dosing guidance, product labeling, and emergency use: FDA.
- World Health Organization (WHO) — technical reports and quality control in pharmaceutical preparations: WHO.
- National Institute of Standards and Technology (NIST) — physical constants and density references for solvents: NIST.
- PubChem / ChemSpider — substance-specific physical properties (density, molecular weight): PubChem.
Use these authorities to validate default density tables, acceptable concentration ranges, and required labeling practices. For institutional calculators, consider review by a licensed pharmacist and IT validation per local health-system policies.
UX considerations for clinician-facing tools
The user interface should prioritize safety and traceability:
- Clear labeling of every input and output with units visible.
- Step-by-step expandable calculation trace for auditability.
- Preset commonly used concentrations and densities with the ability to override for batch-specific values.
- Contextual warnings: tiny volumes (<0.05 mL), volumes > recommended single-dose limits, and non-aqueous solvents needing special handling.
- Accessibility: keyboard navigation, large fonts for critical numbers, and color-blind friendly indicators.
Quality assurance, testing scenarios, and recommended acceptance criteria
Before deploying a free online mg-to-mL calculator for clinical or laboratory use, perform the following tests and document results:
- Unit tests for each formula variant (mg/mL, % w/v, ratio, and density) across a representative range of inputs.
- Boundary tests: zero, extremely small (<0.001 mg), and extremely large (>100000 mg) values.
- Cross-validation: compare outputs against manual calculations and multiple authoritative calculators or spreadsheet templates.
- Performance tests: ensure prompt calculation for batch conversions and concurrent users.
- Usability tests with clinicians and pharmacists to confirm the clarity of steps and warnings.
Frequently asked technical questions
How does temperature influence conversions?
Temperature changes alter density for non-ideal liquids (e.g., ethanol), which affects volume computed from mass. For aqueous solutions at clinical temperatures (15–40 °C) density variability is small but non-negligible when calculating microvolumes. A high-precision calculator can include temperature-corrected density tables sourced from NIST or manufacturer data.
When should concentration be expressed as mg/mL versus % w/v?
Pharmaceutical labels vary: injectable and IV admixture often use mg/mL, topical or compounded products often use % w/v. Internally, calculators should convert any input to mg/mL for unified computation and display both original and converted representations for clarity.
How should the calculator handle compound mixtures and partial composition?
For mixtures where the requested mg refers to one component of multiple active ingredients, the calculator must accept the specific component concentration. For suspensions or emulsions where homogeneity may be variable, include a cautionary statement and recommend mixing before sampling.
Final operational checklist for deploying a free, accurate mg→mL calculator
- Confirm default concentration and density tables against authoritative sources.
- Implement robust input validation and unit conversion logic.
- Display step-by-step calculations with option to download an audit report.
- Include clinical safety warnings for microvolumes and high concentrations.
- Document testing evidence and assign responsible clinical reviewer(s).
- Publish versioning and change log so users can verify the calculator state at time of use.
Further reading and authoritative resources
- United States Pharmacopeia (USP): https://www.usp.org — compounding and labeling standards.
- Food and Drug Administration (FDA): https://www.fda.gov — drug labeling guidance and dosing safety.
- European Medicines Agency (EMA): https://www.ema.europa.eu — quality guidelines for medicinal products.
- World Health Organization (WHO): https://www.who.int — technical reports on pharmaceutical quality.
- NIST Physical Reference Data: https://www.nist.gov — reference densities and thermophysical data.
- PubChem Substance Database: https://pubchem.ncbi.nlm.nih.gov — physico-chemical properties for specific molecules.
By implementing the formulas, validation checks, and user experience design described, an online free mg-to-mL calculator can offer clinicians and laboratory personnel a precise, auditable, and safe tool for routine dosing and formulation tasks. Always cross-check calculator outputs against institutional protocols and pharmacopoeial specifications prior to clinical application.