Accurate OD600 calculations are essential for reproducible bacterial growth measurements in laboratory workflows and diagnostics.
This calculator tool standardizes conversions between absorbance, cell concentration, and dilution factors for cultures accurately
Optical Density (OD600) — Quantification and Normalization Tool
Overview and technical intent
This article defines the engineering, mathematical, and procedural basis for an OD600 calculator optimized as a must-have laboratory tool. It targets microbiologists, process engineers, automation developers, and quality personnel who need rigorous, reproducible conversions between optical density at 600 nm (OD600), actual cell concentration, and associated dilution/volume planning for experiments and production.
Why OD600 calculators are essential for labs and bioprocesses
OD600 is widely used as a rapid proxy of biomass. However, raw absorbance values require calibration, pathlength correction, and conversion factors to produce meaningful cell counts or biomass concentrations. A robust calculator integrates these corrections, stores species-specific calibration coefficients, and outputs actionable volume and dilution instructions.
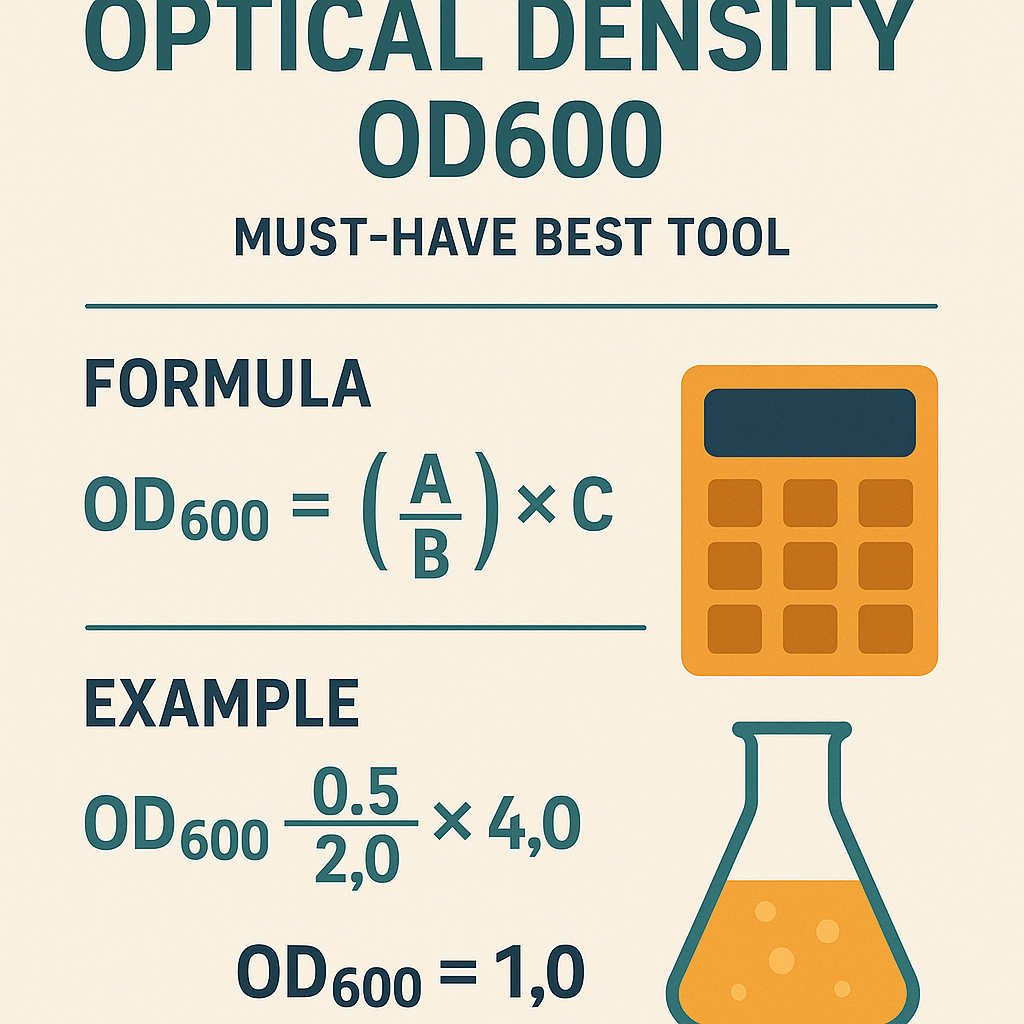
Primary objectives of a rigorous OD600 calculator
- Convert OD600 raw absorbance to cell concentration (cells·mL−1) using validated calibration factors.
- Compensate for microplate or cuvette optical pathlength differences.
- Perform dilution and volume calculations with significant figures and error propagation.
- Estimate growth rate (µ) and doubling time (td) from OD time-series.
- Provide traceable records and link to normative methods for audit and compliance.
Fundamental optical and microbiological formulas
Below are the essential formulas used by the calculator. Each formula is presented in plain HTML and followed by variable definitions and typical values used in lab practice.
Absorbance (Beer–Lambert relation for homogeneous suspensions)
Formula: OD600 = -log10(I / I0)
- I0 = incident light intensity at 600 nm (typical units: arbitrary detector units).
- I = transmitted light intensity at 600 nm.
- Limitations: For dense cultures, multiple scattering invalidates strict linearity of Beer–Lambert; therefore, OD600 linearity typically holds between 0.05 and 0.8 in 1 cm cuvettes.
Concentration conversion using calibration factor
Formula: N = OD600 × K
- N = cell concentration (cells·mL−1).
- OD600 = measured optical density at 600 nm (pathlength-corrected to 1 cm).
- K = calibration factor (cells·mL−1 per OD unit) determined experimentally for strain, medium, and instrument.
- Typical K values: E. coli: 8.0 × 108 cells·mL−1·OD−1; S. cerevisiae: 3.0 × 107 cells·mL−1·OD−1 (see species table).
Simple dilution and mixing (conservation of cells)
Formula: C1 × V1 = C2 × V2
- C1 = initial concentration (cells·mL−1 or OD units).
- V1 = volume of stock required (mL).
- C2 = desired final concentration.
- V2 = final volume.
- Typical use: calculate V1 when preparing a culture at specific OD or cells·mL−1 from a stock.
Specific growth rate and doubling time
Formula (exponential growth): µ = (ln(OD2) − ln(OD1)) / (t2 − t1)
Doubling time: td = ln(2) / µ
- µ = specific growth rate (time−1, e.g., h−1).
- OD1, OD2 = optical density measurements at times t1 and t2 respectively (pathlength-corrected).
- t1, t2 = timepoints (same units, e.g., hours).
- Typical µ for E. coli in rich medium: 0.6–1.2 h−1 (td ≈ 0.58–1.16 hours), depending on conditions.
Microplate pathlength correction
Formula: OD1cm = ODmeas × (1 / l)
- OD1cm = absorbance normalized to a 1 cm pathlength.
- ODmeas = microplate measured absorbance.
- l = effective well pathlength in cm (depends on well geometry and volume).
- Typical l values for clear flat-bottom 96-well plates: 200 µL → l ≈ 0.56 cm; 150 µL → l ≈ 0.42 cm. Therefore pathlength factors ≈ 1.79 and 2.38 respectively.
Calibration and validation: converting OD600 to cells per milliliter reliably
Calibration is mandatory. The calculator must support the creation and storage of calibration curves linking OD600 to direct cell count methods (CFU plating, hemocytometer, or automated cell counters). The stored calibration is used as K in N = OD × K.
Recommended calibration protocol (high level)
- Grow target organism to mid-exponential phase under controlled conditions in target medium.
- Measure OD600 at multiple dilutions using instrument and pathlength identical to planned use.
- Perform parallel direct counts: plate serial dilutions for CFU·mL−1 and/or use a hemocytometer or coulter counter to determine cells·mL−1.
- Plot cells·mL−1 vs OD600 (pathlength-corrected). Perform linear regression in the linear OD range.
- Store slope (K) and correlation coefficient (R2) with metadata (date, strain, medium, instrument).
Extensive tables of common species conversion factors and pathlengths
| Organism | Typical OD600 = 1 → cells·mL−1 (approx.) | Linear OD range (1 cm) | Notes / variance factors |
|---|---|---|---|
| Escherichia coli (K-12, DH5α) | 8.0 × 108 | 0.05 − 0.8 | Depends on strain, medium, and cell size; 5–10×108 reported. |
| Bacillus subtilis | 3.0 × 108 | 0.05 − 0.7 | Tends to scatter more due to cell chains and size heterogeneity. |
| Pseudomonas aeruginosa | 7.0 × 108 | 0.05 − 0.8 | Biofilm-forming strains may clump; calibrate under intended conditions. |
| Saccharomyces cerevisiae (baker's yeast) | 3.0 × 107 | 0.05 − 1.0 | Yeast cells larger; OD→cells relationship different than bacteria. |
| Staphylococcus aureus | 2.5 × 108 | 0.05 − 0.7 | Smaller cocci; calibration required per strain and medium. |
| Plate type/volume | Typical effective pathlength (l, cm) | Pathlength correction factor (1 / l) | Use case |
|---|---|---|---|
| 96-well, flat-bottom, 200 µL | ≈ 0.56 | ≈ 1.79 | Standard high-throughput OD600 on plate readers; correct for pathlength. |
| 96-well, flat-bottom, 150 µL | ≈ 0.42 | ≈ 2.38 | Lower volumes reduce pathlength; apply factor. |
| 384-well, flat-bottom, 50 µL | ≈ 0.15 | ≈ 6.67 | Small volumes require large correction and increase noise. |
| Standard cuvette | 1.00 | 1.00 | Reference pathlength for OD calibration. |
Measurement best practices and error mitigation
- Always blank the instrument with the same medium used for culture measurements (same temperature and container).
- Keep OD600 measurements within the instrument linear range; dilute dense cultures to fall within linear range and apply dilution factor when calculating concentrations.
- When using microplates, perform pathlength correction or use plate reader software with automatic pathlength normalization.
- Record calibration metadata (strain, medium, instrument, date) and maintain traceability for process control.
- Report and propagate uncertainty: instrument reading error, pipetting error, and calibration fit uncertainty must be combined when accuracy is required.
Two complete worked examples with step-by-step solutions
Case 1 — Preparing a culture at target OD from a concentrated stock
Problem statement: You have an E. coli stock measured as OD600 = 2.50 in a cuvette (1 cm pathlength). You need to prepare 50.0 mL of culture at OD600 = 0.10. Determine the required volume of stock (V1) and fresh medium volume to add. Also convert the final OD to cells·mL−1 using a standard calibration factor.
Step-by-step solution:
- Use the dilution formula: C1 × V1 = C2 × V2. Here, C1 = 2.50 OD, C2 = 0.10 OD, V2 = 50.0 mL.
- Solve for V1: V1 = (C2 × V2) / C1 = (0.10 × 50.0) / 2.50.
- Compute: 0.10 × 50.0 = 5.0; 5.0 / 2.50 = 2.0 mL. So V1 = 2.0 mL of stock.
- Volume of medium to add = V2 − V1 = 50.0 − 2.0 = 48.0 mL.
- Convert final OD to cells·mL−1 using K = 8.0 × 108 cells·mL−1·OD−1 (typical for E. coli): N = OD × K = 0.10 × 8.0 × 108 = 8.0 × 107 cells·mL−1.
- If plating for CFU, the expected CFU·mL−1 will often be lower than total cell count due to viability; typically CFU ≈ 0.5–0.9 × total cells depending on conditions — record plating results for calibration.
Final answer: Pipette 2.0 mL of stock into 48.0 mL fresh medium to obtain 50.0 mL at OD600 = 0.10, equivalent to approximately 8.0 × 107 cells·mL−1 for E. coli using the provided calibration factor.
Case 2 — Growth rate and doubling time calculation from OD time-series with pathlength correction
Problem statement: You measure OD in a 96-well plate with 200 µL per well (pathlength ≈ 0.56 cm). Measured OD600 values at two timepoints are: ODmeas,1 = 0.125 at t1 = 30 minutes, and ODmeas,2 = 0.800 at t2 = 210 minutes. Determine the pathlength-corrected OD values, specific growth rate µ (hours−1), and doubling time td (minutes).
Step-by-step solution:
- Pathlength correction factor = 1 / l ≈ 1.79 for 200 µL. Compute OD1cm,1 = 0.125 × 1.79 ≈ 0.2238. OD1cm,2 = 0.800 × 1.79 ≈ 1.432.
- Use the exponential growth formula: µ = (ln(OD2) − ln(OD1)) / (t2 − t1). Convert times to hours. t1 = 30 min = 0.5 h. t2 = 210 min = 3.5 h. Δt = 3.0 h.
- Compute natural logs: ln(1.432) ≈ 0.359; ln(0.2238) ≈ −1.497.
- Compute numerator: 0.359 − (−1.497) = 1.856.
- Compute µ = 1.856 / 3.0 h = 0.6187 h−1.
- Doubling time td = ln(2) / µ = 0.693147 / 0.6187 h ≈ 1.120 h = 67.2 minutes.
- Interpretation: The culture doubled approximately every 67 minutes under the measured conditions. Validate by obtaining multiple intervals during exponential phase and averaging µ values with weighted regression.
Final answer: Pathlength-corrected OD values: ~0.224 and ~1.432. Growth rate µ ≈ 0.619 h−1. Doubling time ≈ 67 minutes.
Uncertainty quantification and propagation
When the calculator provides final cell concentrations and volumes for critical applications (e.g., inoculum preparation for bioreactors or for regulatory reports), propagate uncertainties from the following components:
- Instrument noise and photometric accuracy (typically ±0.005–0.01 OD for bench spectrophotometers).
- Pipetting error for V1/V2 (manufacturer specifications or calibration records).
- Calibration slope uncertainty (standard error from linear regression of cells vs OD).
- Dilution and plating errors for CFU validation.
Propagation example (simplified): If OD measurement uncertainty is ±0.01 and K uncertainty is ±10%, then relative uncertainty in N = OD × K is sqrt((0.01/OD)2 + (0.10)2). For OD = 0.10, relative OD error = 0.01/0.10 = 0.10 (10%), combined relative uncertainty ≈ sqrt(0.102 + 0.102) ≈ 0.141 (14.1%).
Software and UX considerations for an OD600 calculator tool
Designing the calculator interface and data management matters as much as the math. The calculator should:
- Allow user-selectable species/strain with stored calibration coefficients and allow custom calibration uploads (CSV import of calibration curves).
- Support pathlength normalization presets for common plate types and volumes, plus a custom pathlength input.
- Include a “dilute sample” helper: if measured OD exceeds linear range, calculate dilution factor and resulting OD1cm.
- Offer batch processing: convert a column of OD readings to cells·mL−1 and compute µ across sliding windows.
- Export results with metadata (operator, instrument, lot numbers) for traceability.
Recommended UI elements
- Input pane: measured OD, instrument type, pathlength or plate volume, species/strain selection.
- Calibration manager: list calibrations with R2, date, and option to set as default.
- Calculator output: pathlength-corrected OD, cells·mL−1, dilution instructions (V1, V2), and uncertainty estimates.
- Time-series analyzer: upload time-stamped OD series, compute µ and td for selectable intervals.
- Audit log and export (CSV, PDF) with links to referenced normative documents.
Regulatory and normative references
When OD measurements feed into regulated workflows (pharmaceutical development, QC for bioprocesses), refer to normative guidance for instrument calibration, method validation, and traceability. Examples include:
- Zwietering MH, Jongenburger I, Rombouts FM, van 't Riet K. Modeling of the bacterial growth curve. Applied and Environmental Microbiology. 1990;56(6):1875–1881. (Defines growth phases and modeling approaches.)
- CLSI (Clinical and Laboratory Standards Institute) documents for microbiological methods and inoculum preparation (e.g., CLSI M07 for dilution methods). Consult current CLSI catalog for specific method numbers and updates.
- Manufacturer instrument manuals and validation procedures (e.g., Thermo Fisher Scientific spectrophotometer application notes on pathlength and microplate absorbance).
- Good Laboratory Practice (GLP) and ISO 17025 for laboratories performing quantitative measurements—ensure calibration of spectrophotometers is traceable to manufacturer or certified laboratories.
Selected authoritative external resources and application notes
- Thermo Fisher Scientific — Spectrophotometer and microplate reader application notes and guides. https://www.thermofisher.com
- OpenWetWare overview of spectrophotometry and OD measurements. https://openwetware.org
- PubMed/NCBI — for peer-reviewed articles on calibration and growth modelling: https://www.ncbi.nlm.nih.gov
- CLSI — Clinical and Laboratory Standards Institute (method standards and guidelines). https://clsi.org
- NIST — guidance on metrology and instrument calibration relevant to optical measurements. https://www.nist.gov
Practical recommendations for laboratory implementation
- Establish per-strain and per-medium calibration tables and save them in the calculator database with versioning.
- Implement automatic pathlength correction for plate readers; if unavailable, default to cuvette measurements for critical quantitation.
- Document linear range and implement warnings when measured OD falls outside validated range.
- Use multiple replicates and compute mean ± standard deviation for OD and propagated concentration.
- Train personnel to interpret OD vs CFU discrepancies and to perform periodic validation with plating or automated cell counters.
Limitations and caveats
- OD600 is an indirect measurement sensitive to cell size, shape, refractive index, and medium particulates; the same OD can represent different biomass across species or conditions.
- At high densities, multiple scattering invalidates linear conversion; always dilute and back-calc when necessary.
- Calibration factors are instrument- and condition-specific; do not reuse a K factor generated on a different instrument or medium without revalidation.
- CFU counts reflect viable cells while optical methods count both viable and nonviable particles; expect systematic differences.
Maintenance, QA, and auditability
For a calculator embedded into quality systems, ensure:
- Regular instrument performance verification against certified neutral-density filters or standard absorbance references.
- Periodic recalibration of K using fresh cultures, especially when strain or medium changes occur.
- Data retention policies for calibration curves, OD time series, and calculation logs to support audits.
- Version control and electronic signatures for calculator updates that affect conversion logic.
Final technical checklist before deploying the OD600 calculator in routine workflows
- Validate instrument linearity and pathlength calibration.
- Create and store species/strain-specific calibration coefficients with metadata.
- Implement dilution calculators and clear guidance messages for out-of-range readings.
- Include uncertainty propagation and require user acknowledgement for critical transfers to process or regulatory documentation.
- Provide links to normative documents and require training completion for authorized users.
References and further reading
- Zwietering MH, Jongenburger I, Rombouts FM, van 't Riet K. Modeling of the bacterial growth curve. Appl Environ Microbiol. 1990;56(6):1875–1881. https://www.ncbi.nlm.nih.gov/pubmed/16348275
- OpenWetWare — General notes on spectrophotometry and OD measurements: https://openwetware.org/wiki/Spectrophotometry
- Thermo Fisher Scientific — Application notes for spectrophotometers and microplate readers: https://www.thermofisher.com
- CLSI — Standards for microbiology methods and inoculum standardization (consult current documents at https://clsi.org)
- NIST — Reference materials and calibration guidance for optical measurement equipment: https://www.nist.gov
Using an OD600 calculator that enforces calibration, pathlength correction, uncertainty propagation, and traceable metadata greatly reduces variability in experimental and production outcomes. Implement the recommendations here to develop or adopt a best-in-class tool that becomes indispensable in microbiology and bioprocess labs.