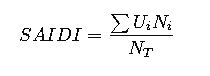Understanding the Calculation of Electrical Work Done by a Galvanic Cell
Electrical work done by a galvanic cell quantifies energy conversion from chemical to electrical form. This article explores detailed calculations and practical applications.
Discover comprehensive formulas, variable explanations, and real-world examples to master the electrical work calculation in galvanic cells.
- Calculate electrical work done by a galvanic cell with E° = 1.10 V and 2 moles of electrons transferred.
- Determine work output for a Zn-Cu galvanic cell operating at 25°C with a cell potential of 1.10 V.
- Find the electrical work done when 3 moles of electrons flow through a cell with E = 0.75 V.
- Compute the maximum electrical work from a galvanic cell with ΔG° = -237 kJ/mol.
Comprehensive Tables of Common Values in Electrical Work Calculations
| Parameter | Symbol | Typical Values | Units | Description |
|---|---|---|---|---|
| Cell Potential (Standard Electrode Potential) | E° | 0.00 to 3.00 | Volts (V) | Voltage difference between electrodes under standard conditions |
| Number of Electrons Transferred | n | 1 to 6 | unitless | Electrons involved in the redox reaction |
| Faraday’s Constant | F | 96485 | Coulombs per mole (C/mol) | Charge per mole of electrons |
| Gibbs Free Energy Change | ΔG | -237000 to 0 | Joules (J) | Maximum reversible work obtainable from the cell |
| Temperature | T | 273 to 373 | Kelvin (K) | Operating temperature of the cell |
| Universal Gas Constant | R | 8.314 | J/(mol·K) | Constant used in thermodynamic equations |
| Reaction Quotient | Q | 0.01 to 100 | unitless | Ratio of product and reactant activities |
| Electrical Work Done | W | Variable | Joules (J) | Energy output as electrical work |
Fundamental Formulas for Calculating Electrical Work Done by a Galvanic Cell
Calculating the electrical work done by a galvanic cell involves thermodynamic and electrochemical principles. The key relationship connects Gibbs free energy change (ΔG) with electrical work (W) and cell potential (E).
1. Relationship Between Gibbs Free Energy and Electrical Work
The maximum non-expansion work (electrical work) obtainable from a galvanic cell is equal to the negative change in Gibbs free energy:
Where:
- W = electrical work done (Joules, J)
- ΔG = Gibbs free energy change (Joules, J)
2. Gibbs Free Energy Change and Cell Potential
The Gibbs free energy change is related to the cell potential by the equation:
Where:
- n = number of moles of electrons transferred (unitless)
- F = Faraday’s constant (96485 C/mol)
- E = cell potential (Volts, V)
This formula assumes standard conditions unless otherwise specified.
3. Nernst Equation for Non-Standard Conditions
Cell potential under non-standard conditions is calculated using the Nernst equation:
Where:
- E° = standard cell potential (V)
- R = universal gas constant (8.314 J/mol·K)
- T = temperature in Kelvin (K)
- Q = reaction quotient (unitless)
4. Calculating Electrical Work from Cell Potential
Combining the above, the electrical work done by the cell is:
Note that this represents the maximum electrical work obtainable under reversible conditions.
5. Additional Considerations: Temperature and Reaction Quotient
Since cell potential depends on temperature and concentrations, the electrical work can be adjusted accordingly:
This formula accounts for deviations from standard conditions, providing accurate work calculations in practical scenarios.
Detailed Explanation of Variables and Their Typical Values
- n (Number of Electrons Transferred): This integer value depends on the redox reaction. For example, in the Zn-Cu cell, n = 2.
- F (Faraday’s Constant): A fundamental constant representing the charge per mole of electrons, 96485 C/mol.
- E° (Standard Cell Potential): Measured in volts, it is the potential difference under standard conditions (1 M concentration, 1 atm pressure, 25°C).
- E (Cell Potential): Actual potential under given conditions, calculated via the Nernst equation.
- R (Universal Gas Constant): 8.314 J/mol·K, used in thermodynamic calculations.
- T (Temperature): Absolute temperature in Kelvin; standard is 298 K (25°C).
- Q (Reaction Quotient): Ratio of product to reactant activities, influencing cell potential.
- ΔG (Gibbs Free Energy Change): Indicates spontaneity and maximum work obtainable; negative values indicate spontaneous reactions.
Real-World Applications: Case Studies in Electrical Work Calculation
Case Study 1: Electrical Work Done by a Zn-Cu Galvanic Cell
Consider a galvanic cell composed of a zinc electrode and a copper electrode. The standard cell potential (E°) is 1.10 V, and the reaction involves the transfer of 2 moles of electrons. Calculate the maximum electrical work done by this cell at 25°C.
Step 1: Identify Variables
- n = 2 (electrons transferred)
- E° = 1.10 V
- F = 96485 C/mol
- T = 298 K (25°C)
Step 2: Calculate Electrical Work
Using the formula:
Substituting values:
The maximum electrical work done by the Zn-Cu galvanic cell is approximately 212.3 kJ.
Case Study 2: Effect of Concentration on Electrical Work Using the Nernst Equation
Consider the same Zn-Cu cell, but now the copper ion concentration is 0.01 M and zinc ion concentration is 1 M at 25°C. Calculate the electrical work done.
Step 1: Calculate Reaction Quotient (Q)
The half-reactions are:
- Zn → Zn2+ + 2e–
- Cu2+ + 2e– → Cu
Reaction quotient Q is:
Step 2: Calculate Cell Potential Using Nernst Equation
Substitute values:
Calculate ln(100) = 4.605
Calculate the fraction:
Then:
Step 3: Calculate Electrical Work
The electrical work done under these non-standard conditions is approximately 201.0 kJ, slightly less than under standard conditions due to concentration effects.
Additional Insights and Practical Considerations
Understanding the calculation of electrical work done by galvanic cells is crucial for designing efficient batteries, fuel cells, and electrochemical sensors. The maximum work output is limited by thermodynamic constraints, and real systems often operate below this ideal due to irreversibilities.
Temperature, concentration, and pressure variations significantly impact cell potential and thus electrical work. Accurate modeling requires incorporating these factors via the Nernst equation and related thermodynamic principles.
- Battery Design: Optimizing electrode materials and electrolyte concentrations to maximize electrical work.
- Fuel Cells: Calculating work output to assess efficiency and power density.
- Corrosion Prevention: Understanding galvanic interactions to minimize unwanted electrical work causing material degradation.