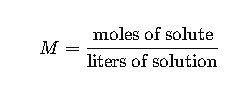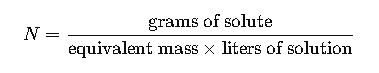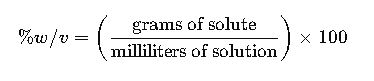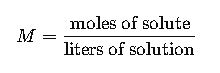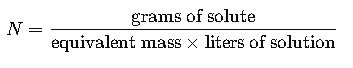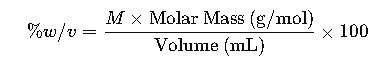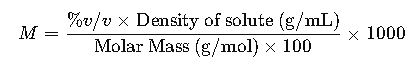Understanding concentration calculations is essential across chemistry, biology, and environmental science, ensuring precise solution preparations. This guide explores Molarity, Normality, % w/v, and % v/v, including formulas, examples, and tables.
Concentration Calculator
What is Molarity?
Molarity (M) = moles of solute / liters of solution.
What is Normality?
Normality (N) = equivalent moles of solute / liters of solution. Requires equivalent weight.
What are % w/v and % v/v?
% w/v = grams of solute per 100 mL solution. % v/v = mL of solute per 100 mL solution.
1. Concentration Units and Their Formulas
1.1 Molarity (M)
- Definition: Molarity is the number of moles of solute dissolved in one liter of solution.
- Formula:
- Variables:
- Moles of solute: Calculated as mass of solute (g) divided by molar mass (g/mol).
- Liters of solution: Total volume of the solution in liters.
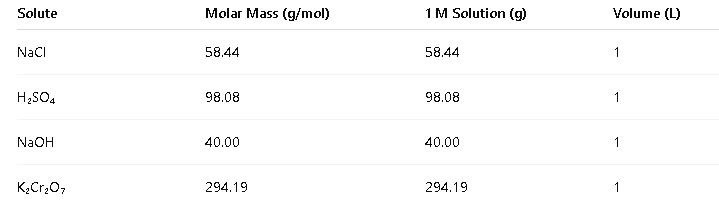
- Common Values:
1.2 Normality (N)
- Definition: Normality is the number of gram equivalents of solute per liter of solution.
- Formula:
- Variables:
- Grams of solute: Mass of the solute in grams.
- Equivalent mass: Molar mass divided by the valence (n-factor) of the solute.
- Liters of solution: Total volume of the solution in liters.
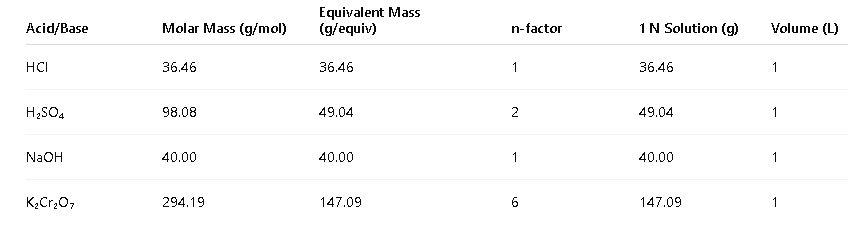
- Common Values:
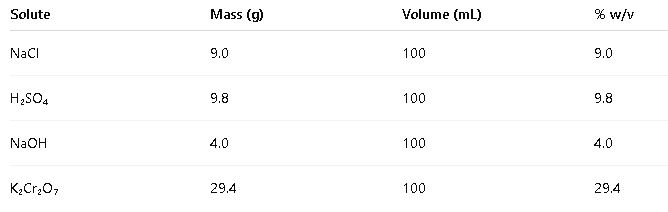
1.3 Weight/Volume Percent (% w/v)
- Definition: Weight/volume percent is the mass of solute in grams per 100 milliliters of solution.
- Formula:
- Variables:
- Grams of solute: Mass of the solute in grams.
- Milliliters of solution: Total volume of the solution in milliliters.
- Common Values:
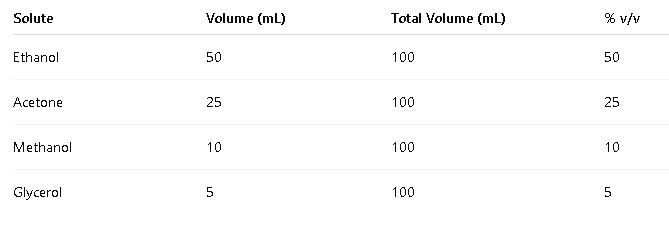
1.4 Volume/Volume Percent (% v/v)
- Definition: Volume/volume percent is the volume of solute in milliliters per 100 milliliters of solution.
- Formula:
- Variables:
- Milliliters of solute: Volume of the solute in milliliters.
- Milliliters of solution: Total volume of the solution in milliliters.
- Common Values:
2. Detailed Explanation of Formulas
2.1 Molarity (M)
- Formula:
- Explanation:
- Moles of solute: Calculated by dividing the mass of the solute (in grams) by its molar mass (in g/mol).
- Liters of solution: The total volume of the solution, which includes both the solute and the solvent.
- Example:
- To prepare 1 liter of 1 M NaCl solution:
- Calculate moles of NaCl:
- Moles = 1 M × 1 L = 1 mole
- Calculate mass of NaCl:
- Mass = moles × molar mass = 1 mole × 58.44 g/mol = 58.44 g
- Dissolve 58.44 g of NaCl in enough water to make the total volume 1 liter.
- Calculate moles of NaCl:
- To prepare 1 liter of 1 M NaCl solution:
2.2 Normality (N)
- Formula:
- Explanation:
- Grams of solute: The mass of the solute in grams.
- Equivalent mass: Calculated by dividing the molar mass of the solute by its valence (n-factor), which represents the number of reactive units (e.g., H⁺ ions for acids, OH⁻ ions for bases).
- Liters of solution: The total volume of the solution in liters.
- Example:
- To prepare 1 liter of 1 N H₂SO₄ solution:
- Calculate equivalent mass of H₂SO₄:
- Equivalent mass = molar mass / n-factor = 98.08 g/mol / 2 = 49.04 g/equiv
- Calculate grams of H₂SO₄:
- Grams = N × equivalent mass × volume = 1 N × 49.04 g/equiv × 1 L = 49.04 g
- Dissolve 49.04 g of H₂SO₄ in enough water to make the total volume 1 liter.
- Calculate equivalent mass of H₂SO₄:
- To prepare 1 liter of 1 N H₂SO₄ solution:
2.3 Volume/Volume Percent (% v/v)
- Formula:
- Explanation:
- Milliliters of solute: Volume of the solute added to the solution.
- Milliliters of solution: Total final volume of the solution after adding the solute.
- Example:
- To prepare 100 mL of 70% v/v ethanol solution:
- Volume of ethanol needed = (70/100) × 100 mL = 70 mL
- Add ethanol to water until the total solution volume = 100 mL.
- To prepare 100 mL of 70% v/v ethanol solution:
3. Interconversion Between Concentration Units
Sometimes, it is necessary to convert between molarity, normality, and percent concentrations. The relationships depend on the molecular weight, density, and reaction equivalents.
3.1 Molarity to Normality
N=M×n
Where:
- N = Normality (eq/L)
- M = Molarity (mol/L)
- n = n-factor (number of reactive units per molecule)
Example:
- For H₂SO₄, M = 1 M, n-factor = 2 → N = 1 × 2 = 2 N.
3.2 Molarity to % w/v
- For 1 M NaCl in 100 mL:
- Mass = 1 × 58.44 g/mol × 0.1 L = 5.844 g
- % w/v = (5.844 / 100 mL) × 100 = 5.844% w/v
3.3 % v/v to Molarity
- Converts % v/v (volume) into molarity (mol/L) considering density.
4. Real-World Applications of Concentration Calculations
Concentration calculations are essential in laboratory work, pharmaceuticals, industrial chemistry, and environmental monitoring.
4.1 Pharmaceutical Industry: Drug Formulation
Case Study: Preparing a saline solution for intravenous (IV) therapy.
- Target: 0.9% w/v NaCl solution in 500 mL.
- Calculation:
- Mass of NaCl = (0.9 / 100) × 500 mL = 4.5 g
- Dissolve 4.5 g NaCl in water to make final volume 500 mL.
- Application: Ensures isotonicity for patient safety.
4.2 Environmental Chemistry: Acid Neutralization
Case Study: Neutralizing sulfuric acid spill using sodium hydroxide.
- Spill: 2 L of 1 N H₂SO₄
- Goal: Determine grams of NaOH needed for neutralization.
- Reaction: H₂SO₄ + 2 NaOH → Na₂SO₄ + 2 H₂O
- Calculation:
- Normality of H₂SO₄ = 1 N → Eq = 1 eq/L × 2 L = 2 eq
- NaOH required = 1 g/eq × 2 eq = 80 g (since molar mass = 40 g/mol, 1 eq per mole)
- Application: Ensures safe disposal of acid in compliance with environmental regulations.
5. Practical Tables for Quick Reference
5.1 Molarity Conversion Table
| Solute | Molar Mass (g/mol) | 1 M (g/L) | 0.1 M (g/L) | 0.01 M (g/L) |
|---|---|---|---|---|
| NaCl | 58.44 | 58.44 | 5.844 | 0.5844 |
| HCl | 36.46 | 36.46 | 3.646 | 0.3646 |
| KOH | 56.11 | 56.11 | 5.611 | 0.5611 |
| H₂SO₄ | 98.08 | 98.08 | 9.808 | 0.9808 |
5.2 Normality Reference Table
| Solute | Molar Mass | n-factor | 1 N (g/L) | 0.5 N (g/L) | 0.1 N (g/L) |
|---|---|---|---|---|---|
| HCl | 36.46 | 1 | 36.46 | 18.23 | 3.646 |
| H₂SO₄ | 98.08 | 2 | 49.04 | 24.52 | 4.904 |
| NaOH | 40.00 | 1 | 40.00 | 20.00 | 4.00 |
| K₂Cr₂O₇ | 294.19 | 6 | 49.03 | 24.515 | 4.903 |
5.3 % w/v Quick Reference
| Solution | % w/v | Mass (g) for 100 mL | Mass (g) for 1 L |
|---|---|---|---|
| NaCl | 0.9 | 0.9 | 9.0 |
| Glucose | 5 | 5 | 50 |
| NaOH | 1 | 1 | 10 |
| KCl | 10 | 10 | 100 |
5.4 % v/v Quick Reference
| Solution | % v/v | Volume Solute (mL) / 100 mL |
|---|---|---|
| Ethanol | 70 | 70 |
| Methanol | 10 | 10 |
| Acetone | 25 | 25 |
| Glycerol | 5 | 5 |
6. Tips for Accurate Solution Preparation
- Always measure solutes using calibrated balances.
- Use volumetric flasks to achieve precise solution volumes.
- Consider temperature effects, as solution volume may expand or contract.
- When diluting concentrated acids/bases, always add acid to water, never the reverse.
- Record all calculations and measurements for traceability.