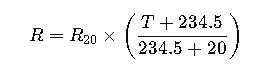Understanding the Calculation of Serial Dilutions: A Technical Deep Dive
Serial dilution calculation is a fundamental technique in quantitative biology and chemistry. It involves systematically reducing concentration to achieve precise, measurable results.
This article explores the mathematical foundations, common values, and real-world applications of serial dilution calculations. Readers will gain expert-level insights and practical knowledge.
- Calculate the final concentration after 5 serial 1:10 dilutions starting from 1 M solution.
- Determine the volume of stock solution needed to prepare 100 mL of 1:1000 diluted solution.
- Find the dilution factor when 50 µL of stock is diluted to 5 mL total volume.
- Explain how to prepare a serial dilution series from 10-1 to 10-6 with 1 mL steps.
Comprehensive Tables of Common Serial Dilution Values
| Dilution Factor (DF) | Stock Volume (mL) | Diluent Volume (mL) | Total Volume (mL) | Resulting Concentration (Relative to Stock) | Log10 Dilution |
|---|---|---|---|---|---|
| 1:2 | 1 | 1 | 2 | 0.5 | -0.301 |
| 1:5 | 1 | 4 | 5 | 0.2 | -0.699 |
| 1:10 | 1 | 9 | 10 | 0.1 | -1.000 |
| 1:20 | 1 | 19 | 20 | 0.05 | -1.301 |
| 1:50 | 1 | 49 | 50 | 0.02 | -1.699 |
| 1:100 | 1 | 99 | 100 | 0.01 | -2.000 |
| 1:200 | 1 | 199 | 200 | 0.005 | -2.301 |
| 1:500 | 1 | 499 | 500 | 0.002 | -2.699 |
| 1:1000 | 1 | 999 | 1000 | 0.001 | -3.000 |
| 1:10,000 | 1 | 9,999 | 10,000 | 0.0001 | -4.000 |
| 1:100,000 | 1 | 99,999 | 100,000 | 0.00001 | -5.000 |
These values represent the most frequently used dilution factors in laboratory settings, ranging from simple 1:2 dilutions to extremely dilute 1:100,000 solutions. The log10 dilution column is critical for plotting and interpreting data in microbiology and biochemistry.
Mathematical Formulas for Serial Dilution Calculations
Serial dilution calculations rely on a set of fundamental formulas that relate volumes, concentrations, and dilution factors. Understanding each variable and its typical values is essential for accurate preparation and analysis.
Basic Dilution Factor Formula
The dilution factor (DF) is defined as the ratio of the final volume (Vf) to the volume of the stock solution (Vs):
- DF: Dilution factor (unitless), e.g., 10 for a 1:10 dilution.
- Vf: Final total volume after dilution (mL or µL).
- Vs: Volume of the stock solution taken for dilution (mL or µL).
For example, if 1 mL of stock is diluted to 10 mL total volume, DF = 10/1 = 10.
Concentration After Dilution
The concentration of the diluted solution (Cd) is related to the initial concentration (Ci) and the dilution factor:
- Ci: Initial concentration of stock solution (mol/L, CFU/mL, etc.).
- Cd: Concentration after dilution.
This formula assumes perfect mixing and no volume loss.
Serial Dilution Concentration After n Steps
When performing multiple serial dilutions with the same dilution factor, the concentration after n steps is:
- Cn: Concentration after n serial dilutions.
- n: Number of dilution steps.
For example, after 3 serial 1:10 dilutions, the concentration is Ci / 103 = Ci / 1000.
Volume of Stock Solution Required for Desired Dilution
To prepare a specific diluted volume (Vf) at a desired dilution factor (DF), the volume of stock solution (Vs) needed is:
The volume of diluent (Vd) is then:
- Vd: Volume of diluent (e.g., buffer, water).
Logarithmic Representation of Dilution
In microbiology and analytical chemistry, dilutions are often expressed logarithmically:
This is useful for plotting dilution series on log scales and interpreting results such as bacterial counts.
Detailed Real-World Examples of Serial Dilution Calculations
Example 1: Preparing a 1:1000 Dilution for Enzyme Assay
A biochemist needs to prepare 10 mL of a 1:1000 dilution from a 2 M stock enzyme solution to perform an activity assay. The goal is to calculate the volume of stock and diluent required and the final concentration.
- Given: Ci = 2 M, DF = 1000, Vf = 10 mL
Step 1: Calculate volume of stock solution:
Step 2: Calculate volume of diluent:
Step 3: Calculate final concentration:
The biochemist should pipette 10 µL of the 2 M stock into 9.99 mL of buffer to obtain 10 mL of 0.002 M enzyme solution.
Example 2: Serial Dilution for Microbial Colony Counting
A microbiologist wants to perform serial 1:10 dilutions to estimate bacterial concentration in a sample. Starting with 1 mL of the original culture, the goal is to prepare dilutions from 10-1 to 10-6 and calculate the expected concentration at each step.
- Initial concentration (Ci): 1 × 109 CFU/mL
- Dilution factor per step: 10
- Number of steps: 6
Step 1: Concentration after each dilution step:
| Dilution Step (n) | Dilution Factor (DFn) | Concentration (CFU/mL) |
|---|---|---|
| 0 (Stock) | 1 | 1 × 109 |
| 1 | 10 | 1 × 108 |
| 2 | 102 = 100 | 1 × 107 |
| 3 | 103 = 1,000 | 1 × 106 |
| 4 | 104 = 10,000 | 1 × 105 |
| 5 | 105 = 100,000 | 1 × 104 |
| 6 | 106 = 1,000,000 | 1 × 103 |
Step 2: Preparation protocol for each dilution:
- Take 1 mL of the previous dilution and add 9 mL of sterile diluent.
- Mix thoroughly before proceeding to the next dilution.
Step 3: Use the 10-6 dilution to plate and count colonies, then multiply by the dilution factor to estimate original concentration.
Additional Considerations and Best Practices
Accurate serial dilution requires precise pipetting and thorough mixing to ensure homogeneity. Errors in volume measurement can propagate exponentially through serial steps, leading to significant inaccuracies.
When working with very small volumes (e.g., microliters), use calibrated micropipettes and consider preparing intermediate dilutions to minimize pipetting errors.
- Always use sterile equipment to avoid contamination, especially in microbiological applications.
- Label each dilution tube clearly to prevent mix-ups.
- Record all volumes and concentrations meticulously for reproducibility.
- Consider the stability of the analyte; some compounds degrade upon dilution or over time.
Useful External Resources for Serial Dilution Calculations
- NCBI: Serial Dilution Techniques in Microbiology
- Sigma-Aldrich: Serial Dilution Protocols
- Thermo Fisher Scientific: How to Perform a Serial Dilution
- NCBI Bookshelf: Dilution and Concentration Calculations
Mastering serial dilution calculations is essential for experimental accuracy in many scientific disciplines. This article provides the technical foundation and practical examples to enhance your proficiency in this critical laboratory skill.