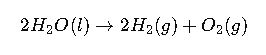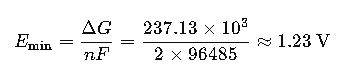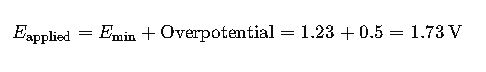Electrolysis is an essential electrochemical process that decomposes compounds, primarily water, into hydrogen and oxygen. The minimum voltage required for electrolysis critically affects process efficiency, feasibility, and real-world industrial applications.
Minimum Voltage for Electrolysis Calculator
Theoretical Minimum Voltage for Electrolysis
The theoretical minimum voltage required for electrolysis is derived from the Gibbs free energy change (ΔG) of the reaction. For the electrolysis of water, the reaction is:
The Gibbs free energy change for this reaction at standard conditions (25°C, 1 atm, 1 M concentrations) is approximately 237.13 kJ/mol. This corresponds to a theoretical minimum voltage of about 1.23 V.
However, this is an idealized value. In practical applications, additional factors such as overpotentials and internal resistances necessitate higher applied voltages.
Overpotentials and Practical Voltage Requirements
Overpotentials are additional voltages required to overcome kinetic barriers at the electrodes during electrolysis. These include:
- Hydrogen Evolution Reaction (HER) Overpotential: Occurs at the cathode during hydrogen production.
- Oxygen Evolution Reaction (OER) Overpotential: Occurs at the anode during oxygen production.
The combined overpotentials for both reactions typically range from 0.4 to 0.6 V, depending on factors such as electrode material, electrolyte composition, and temperature. Therefore, the practical voltage required for water electrolysis is often between 1.6 and 1.8 V.
Factors Influencing Minimum Voltage
Several factors influence the minimum voltage required for electrolysis:
- Electrode Material: Materials with lower overpotentials reduce the required voltage.
- Electrolyte Composition: The type and concentration of the electrolyte affect the conductivity and overpotentials.
- Temperature: Higher temperatures can decrease overpotentials and increase reaction rates.
- Current Density: Higher current densities can lead to increased overpotentials due to factors like bubble formation and electrode degradation.
Real-World Applications and Voltage Considerations
1. Proton Exchange Membrane (PEM) Electrolyzers
PEM electrolyzers operate at voltages ranging from 1.8 to 2.2 V. These systems offer high efficiency and rapid response times, making them suitable for applications like renewable energy storage and fuel cell integration.
2. Alkaline Electrolyzers
Alkaline electrolyzers typically require voltages between 1.6 and 1.8 V. They are widely used in industrial hydrogen production due to their cost-effectiveness and scalability.
3. High-Temperature Electrolysis
In high-temperature electrolysis (HTE), such as solid oxide electrolyzers, the required voltage can be lower than in low-temperature systems due to the thermal energy supplied to the system. HTE systems can operate at voltages as low as 1.2 V under optimal conditions.
Example Calculations
Example 1: Water Electrolysis at Standard Conditions
Given:
- ΔG = 237.13 kJ/mol
Calculation:
Conclusion:
The theoretical minimum voltage required for the electrolysis of water at standard conditions is approximately 1.23 V.
Example 2: Practical Electrolysis with Overpotentials
Given:
- Theoretical voltage = 1.23 V
- Total overpotential = 0.5 V
Calculation:
Conclusion:
In practical applications, an applied voltage of approximately 1.73 V is required to achieve efficient electrolysis of water.
Extensive Table of Common Electrolysis Voltages
| Electrolyte / Reaction | Temperature (°C) | Theoretical Voltage (V) | Typical Practical Voltage (V) | Notes |
|---|---|---|---|---|
| Water (neutral pH) | 25 | 1.23 | 1.6 – 1.8 | Standard water electrolysis |
| Water + KOH 1M | 25 | 1.23 | 1.55 – 1.75 | Alkaline electrolyte lowers resistance |
| Water + H₂SO₄ 0.5M | 25 | 1.23 | 1.6 – 1.8 | Acidic medium, efficient for lab-scale electrolysis |
| Sodium Chloride Solution | 25 | 2.0 | 2.2 – 2.5 | Chlor-alkali process; chlorine evolution at anode |
| Water (high-temperature, 700°C) | 700 | 1.2 | 1.2 – 1.4 | Solid oxide electrolyzers, high efficiency |
| Copper Electroplating | 25 | 0.34 | 0.5 – 0.8 | Electrodeposition of copper, industrial scale |
| Zinc Electroplating | 25 | 0.76 | 1.0 – 1.2 | Electroplating or battery applications |
| Aluminum Electrolysis (cryolite bath) | 960 | 2.0 – 2.5 | 4.0 – 5.0 | High-temperature industrial process, high energy input |
Explanation of Table Values:
- Theoretical Voltage: Minimum voltage required if no energy losses occurred.
- Practical Voltage: Realistic voltage applied considering overpotentials, electrolyte conductivity, and electrode efficiency.
- Notes: Highlights industrial, laboratory, or high-temperature processes.
Key Variables Influencing Electrolysis Voltage
Understanding the factors that determine voltage requirements is essential for designing and optimizing electrolysis systems.
- Gibbs Free Energy (ΔG):
Determines the minimum energy required for a chemical reaction. Higher ΔG → higher voltage. - Number of Electrons Transferred (n):
Directly proportional to energy per mole of electrons. Reactions with more electrons require more voltage. - Faraday Constant (F):
Represents charge per mole of electrons (≈96485 C/mol). Fundamental in calculating energy requirements. - Temperature:
Higher temperatures can reduce the energy required for breaking bonds, slightly lowering the needed voltage. - Electrolyte Concentration and Conductivity:
Conductive electrolytes reduce resistance and overpotential, enabling lower applied voltages. - Electrode Material and Surface Area:
Catalytic materials reduce overpotential; larger surface area improves efficiency.
Real-World Example 1: Industrial Hydrogen Production with Alkaline Electrolyzers
Scenario:
A factory wants to produce hydrogen using an alkaline electrolyzer with a 30% KOH solution at 25°C.
Analysis:
- Theoretical minimum voltage: 1.23 V
- Typical overpotentials for hydrogen and oxygen reactions in alkaline solutions: 0.3 – 0.5 V
- Applied voltage: 1.55 – 1.75 V
Outcome:
- Hydrogen is efficiently produced at the cathode; oxygen at the anode.
- Energy efficiency can reach 65–70% depending on system design.
- Temperature management is essential because electrolysis generates heat.
Insights:
Optimizing electrode catalysts (e.g., nickel or platinum-coated electrodes) reduces overpotentials, decreasing energy costs.
Real-World Example 2: Water Electrolysis for Renewable Energy Storage
Scenario:
A solar farm wants to store excess energy as hydrogen using proton exchange membrane (PEM) electrolyzers.
Analysis:
- Standard theoretical voltage: 1.23 V
- Overpotential in PEM systems: 0.4 – 0.6 V
- Applied voltage: 1.8 – 2.2 V depending on current density and temperature
- Temperature control is crucial: PEM membranes degrade above 80°C
Outcome:
- Hydrogen production provides a method of energy storage for times of low sunlight.
- PEM systems are preferred for dynamic load response due to rapid startup/shutdown capabilities.
Insights:
Careful monitoring of voltage, current density, and water purity is critical to avoid membrane damage and maintain efficiency.
Factors for Optimizing Minimum Voltage in Practice
- Electrode Catalysts:
- Platinum, iridium, or nickel alloys significantly reduce overpotentials.
- Electrolyte Choice:
- Acidic vs alkaline media: acid electrolytes improve efficiency in PEM; alkaline is cost-effective for large-scale production.
- Temperature Control:
- Higher temperatures generally improve kinetics but can increase membrane wear in PEM systems.
- Current Density Optimization:
- Low current densities reduce overpotentials, but very low currents may be inefficient for large-scale hydrogen production.
- System Design:
- Shorter distances between electrodes reduce resistive losses.
- Proper water circulation prevents gas accumulation and local resistance increases.