Accurate conversion from percentage concentration to molarity is vital across analytical chemistry, biochemistry, pharmaceutical sciences, and industry. Professionals convert percentage-based formulations into molarity to standardize procedures, improve reproducibility, and ensure compliance with regulations.
Percentage Concentration → Molarity Calculator
Convert % concentration (w/w or w/v) into molarity (mol·L⁻¹). Includes density option for % w/w and step-by-step output.
Which formula is used for % w/w → M
What about % w/v?
What density should I use?
Formulas and derivation
M = (percent × density × 10) / molar_mass.
For % w/v: mass per 100 mL so grams per L = percent × 10 → M = (percent × 10) / molar_mass.
Comprehensive Reference Tables for Percentage Concentration to Molarity
The following tables provide commonly used conversions for different solutes, assuming a solution density close to water (1 g/mL at room temperature) unless otherwise specified. For rigorous calculations, density corrections must always be applied.
Table 1. Conversion of % w/v (grams of solute per 100 mL solution) to molarity (mol/L)
| Solute | Molar Mass (g/mol) | 1% w/v (M) | 2% w/v (M) | 5% w/v (M) | 10% w/v (M) | 20% w/v (M) |
|---|---|---|---|---|---|---|
| Sodium chloride (NaCl) | 58.44 | 0.171 | 0.342 | 0.855 | 1.71 | 3.42 |
| Potassium chloride (KCl) | 74.55 | 0.134 | 0.268 | 0.671 | 1.34 | 2.68 |
| Glucose (C₆H₁₂O₆) | 180.16 | 0.056 | 0.111 | 0.278 | 0.556 | 1.11 |
| Sucrose (C₁₂H₂₂O₁₁) | 342.30 | 0.029 | 0.058 | 0.146 | 0.292 | 0.584 |
| Hydrochloric acid (HCl, gas in solution)* | 36.46 | 0.274 | 0.548 | 1.37 | 2.74 | 5.48 |
| Acetic acid (CH₃COOH) | 60.05 | 0.167 | 0.333 | 0.833 | 1.67 | 3.33 |
*For strong acids and bases, industrial grades are often expressed in % w/w, not % w/v. Density corrections are necessary.
Table 2. Conversion of % w/w (grams of solute per 100 g solution) to molarity (mol/L)
This calculation requires solution density. Values below assume typical densities from chemical handbooks (CRC Handbook of Chemistry and Physics, NIST data).
| Solute | Molar Mass (g/mol) | Density at 25 °C (g/mL) | 10% w/w (M) | 20% w/w (M) | 30% w/w (M) | 40% w/w (M) |
|---|---|---|---|---|---|---|
| Sodium hydroxide (NaOH) | 40.00 | 1.11 | 2.78 | 5.55 | 8.33 | 11.1 |
| Sulfuric acid (H₂SO₄) | 98.08 | 1.14 | 1.16 | 2.27 | 3.48 | 4.77 |
| Hydrochloric acid (HCl, 37% w/w, ρ=1.19 g/mL) | 36.46 | 1.19 | 3.25 (at 10%) | 6.50 (20%) | 9.75 (30%) | 12.0 (37%) |
| Nitric acid (HNO₃, ρ=1.51 g/mL at 70% w/w) | 63.01 | 1.42 (50%) | 7.83 | 15.7 | 23.5 | 31.3 |
Table 3. Quick Reference for Glucose (% w/v to molarity)
| % w/v | Molarity (M) |
|---|---|
| 0.1% | 0.0056 |
| 0.5% | 0.0278 |
| 1% | 0.0556 |
| 5% | 0.278 |
| 10% | 0.556 |
| 25% | 1.39 |
This table is commonly used in cell culture media and pharmaceutical solutions.
Core Formulas for Percentage Concentration to Molarity
Several scenarios exist depending on whether the concentration is given as % w/v, % w/w, or % v/v. Below are the general equations used.
1. From % w/v to Molarity
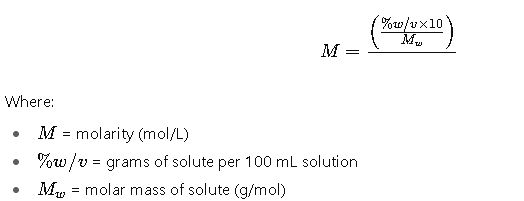
Explanation:
- Multiply % w/v by 10 to convert from 100 mL to 1 L.
- Divide by the molar mass to obtain moles per liter.
2. From % w/w to Molarity
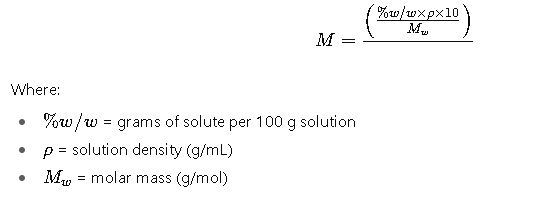
Explanation:
- Multiply % w/w by density × 10 (because 100 g solution / density → volume in mL, scaled to 1 L).
- Divide by molar mass.
3. From % v/v to Molarity
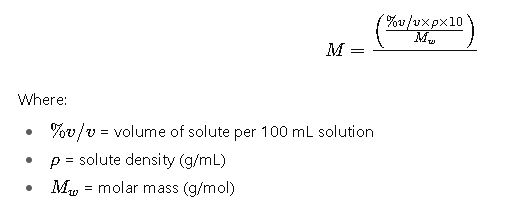
Typical examples: ethanol, acetic acid, or isopropanol in aqueous solutions.
4. Adjusted Formula Using Solution Density (Generalized)
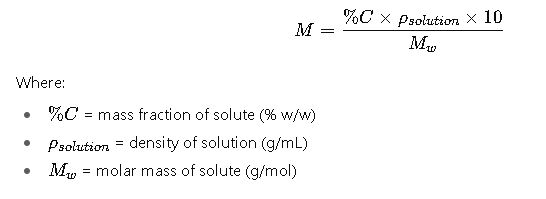
Detailed Variable Explanations
- % w/v: Grams of solute per 100 mL of solution. Common in biological buffers.
- % w/w: Grams of solute per 100 g of solution. Standard in industrial chemicals.
- % v/v: Volume of solute per 100 mL of solution. Used in alcohol concentrations.
- Density (ρ): Required for w/w and v/v conversions. Values are available in NIST databases.
- Molar Mass (Mₙ): Fixed property of each compound, obtained from IUPAC tables.
Real-World Applications of Percentage Concentration to Molarity
The conversion of percentage concentration to molarity is not just an academic exercise; it underpins many real-world scenarios where accuracy determines product quality, regulatory compliance, or even patient safety. Below are detailed applications across different industries.
Application 1. Pharmaceutical Industry – Preparing Intravenous Solutions
In hospitals and pharmaceutical laboratories, intravenous (IV) fluids and injectable medications are often described by percentage concentration, while pharmacokinetic and dosing calculations require molarity.
Case Example: Sodium chloride infusion, labeled as 0.9% w/v (normal saline).
- Clinicians know this corresponds to 9 g of NaCl per liter of solution.
- By understanding the molarity, pharmacologists can calculate the osmolarity of the solution, which is essential for patient safety.
- In this case, the calculated molarity (~0.154 mol/L) corresponds to ~308 mOsm/L, which matches human plasma osmolarity.
Why It Matters:
- Ensures IV fluids are isotonic and do not damage blood cells.
- Aligns with World Health Organization (WHO) guidelines for parenteral solutions.
- Provides a direct connection between formulation (% w/v) and physiological compatibility (molarity).
Application 2. Industrial Chemical Manufacturing – Acids and Bases
In industrial processes, strong acids and bases are typically sold and labeled by % w/w. Engineers and quality assurance teams must convert these to molarity to design reactors, titrations, and neutralization procedures.
Case Example: Hydrochloric acid (HCl) sold at 37% w/w with density ~1.19 g/mL.
- Engineers calculate its molarity to be about 12 M.
- This conversion allows precise dosing in chemical reactors, where stoichiometric ratios depend on molar concentrations rather than percentages.
Why It Matters:
- Enables safe scaling of chemical processes.
- Prevents errors in mixing ratios that could cause runaway reactions.
- Ensures compliance with ISO and ASTM industrial chemical standards.
Application 3. Food and Beverage Technology
Food technologists often work with sugar concentrations in % w/v (such as in soft drinks or syrups) but require molarity to analyze fermentation processes.
Case Example: A 10% w/v glucose syrup used in yeast fermentation.
- Microbiologists studying fermentation kinetics calculate the molarity of glucose to determine substrate-to-biomass conversion rates.
- This allows optimization of alcohol yield in brewing or bioethanol production.
Why It Matters:
- Balances sweetness, fermentation efficiency, and cost.
- Standardizes formulations across production plants.
- Provides comparable data for scientific publications and regulatory documents.
Application 4. Clinical and Diagnostic Laboratories
Diagnostic reagents such as buffers, stains, and enzymes are prepared in percentage concentrations. For enzymatic reactions and spectrophotometric assays, however, molarity is required.
Case Example: Preparation of a 5% w/v acetic acid solution for a laboratory test.
- Lab technicians convert this to molarity to calculate hydrogen ion concentration, ensuring the correct pH environment.
- This is critical in DNA precipitation protocols and histology staining.
Why It Matters:
- Maintains assay reproducibility.
- Avoids errors that could mislead diagnostic interpretations.
- Ensures consistency between labs using international standard operating procedures.
Extended Examples with Step-by-Step Development
To solidify the theory, here are two extended case studies with detailed explanations.
Case Study 1: Preparation of Hydrochloric Acid for Titration
Scenario: A laboratory technician needs to prepare 500 mL of a 1 M HCl solution, but only has concentrated HCl at 37% w/w.
Development:
- Determine the molarity of the stock HCl (about 12 M, based on reference data).
- Apply dilution principles to calculate the volume of concentrated HCl required.
- Carefully dilute the measured volume to exactly 500 mL with deionized water.
Solution and Outcome:
- Approximately 42 mL of concentrated HCl is needed, diluted up to 500 mL.
- The technician now has a standardized 1 M HCl solution, ready for titration experiments.
Practical Notes:
- Always add acid to water, not water to acid.
- Use personal protective equipment, as concentrated HCl is highly corrosive.
- This process aligns with standard laboratory practices recommended by ISO 17025.
Case Study 2: Formulating a Glucose Solution for Cell Culture
Scenario: A biologist needs a 100 mL solution of 0.1 M glucose for cell culture experiments. Stock solution is available as a 10% w/v glucose preparation.
Development:
- Calculate the molarity of the stock 10% glucose solution (~0.556 M).
- Apply dilution ratios to obtain the desired 0.1 M solution.
- Transfer the correct volume of stock solution, dilute with sterile water up to 100 mL.
Solution and Outcome:
- About 18 mL of the stock solution is diluted to 100 mL total.
- The final solution is suitable for maintaining osmotic balance in mammalian cells.
Practical Notes:
- Sterilize by filtration before use in cell culture.
- Confirm glucose concentration with a biochemical analyzer to ensure accuracy.
- This method follows recommendations in the ATCC (American Type Culture Collection) cell culture guide.
Best Practices and Common Pitfalls
When converting percentage concentrations to molarity, professionals must be cautious of several common errors:
- Ignoring density: Especially problematic for concentrated acids and bases, where density deviates significantly from water.
- Confusing % w/v and % w/w: Misinterpretation leads to large calculation errors.
- Not considering hydration states: Compounds like copper sulfate (CuSO₄·5H₂O) have different molar masses than their anhydrous form.
- Rounding errors: In pharmaceutical and diagnostic labs, precision is essential to meet pharmacopeial standards (USP, EP).
Best Practices Include:
- Always reference authoritative data (CRC Handbook, NIST, Merck Index).
- Double-check units before finalizing calculations.
- Document all steps for reproducibility and regulatory audits.
Why This Knowledge Is Essential for Professionals
- Chemists use these conversions daily in solution preparation and stoichiometric calculations.
- Pharmacists rely on molarity conversions to ensure correct drug dosages and IV compatibility.
- Food technologists apply these methods to standardize formulations and fermentation efficiency.
- Engineers in chemical plants depend on accurate molarity data for process control and reactor safety.
By mastering the transition from percentage concentration to molarity, professionals bridge the gap between laboratory labels and scientific calculations.