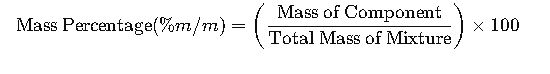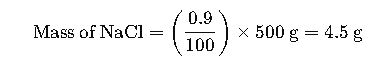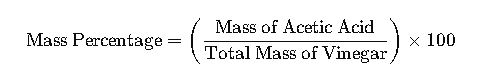Mass percentage (% m/m) quantifies a component’s mass relative to the total mixture accurately.
It is essential in chemistry, industry, and quality control for precise composition and formulation.
Mass Percentage (% m/m) Calculator
Easily calculate the mass percentage of a solute in a solution.
Note: Mass percentage expresses solute mass relative to total solution mass.
Common Mass Percentage Examples
| Solution | Mass % (m/m) |
|---|---|
| Saltwater (sea) | ~3.5% |
| Vinegar (acetic acid) | ~5% |
| Hydrogen peroxide (household) | ~3% |
| Sugar solution (soft drinks) | ~10% |
| Medical saline | 0.9% |
1. Mass Percentage (% m/m): Fundamental Formula and Variables
The mass percentage, commonly referred to as % m/m, is a crucial concept in chemistry and materials science. It quantifies the proportion of a specific component’s mass relative to the total mass of a mixture or compound. The fundamental formula is:
Where:
- Mass of Component: The mass of the specific component of interest within the mixture.
- Total Mass of Mixture: The combined mass of all components in the mixture.
This formula applies universally, encompassing both pure compounds and mixtures, and is pivotal in various applications, including solution preparation, quality control, and material characterization.
2. Common Values and Applications of Mass Percentage
Mass percentages are prevalent in numerous fields. Below is a table illustrating typical mass percentages for various substances in common mixtures:
| Substance | Common Mass Percentage (%) | Application Area |
|---|---|---|
| Sodium chloride (NaCl) | 5% to 10% | Saline solutions in medicine |
| Ethanol in water | 70% to 95% | Disinfectants and sanitizers |
| Sugar in water | 10% to 20% | Food and beverage industry |
| Acetic acid in vinegar | 4% to 8% | Food industry and cleaning |
| Hydrogen peroxide | 3% to 6% | Disinfectants and bleaching |
These values are indicative and can vary based on specific formulations and intended uses.
3. Detailed Explanation of Variables
In the mass percentage formula, each variable plays a critical role:
- Mass of Component: This is the mass of the specific substance within the mixture. It is measured using precise analytical balances to ensure accuracy.
- Total Mass of Mixture: This is the sum of the masses of all components in the mixture. It is essential that all components are accounted for to obtain an accurate total mass.
The accuracy of mass percentage calculations depends on precise measurement and proper accounting of all components in the mixture.
4. Real-World Examples of Mass Percentage Calculations
Example 1: Preparing a Saline Solution
Suppose a laboratory technician needs to prepare 500 mL of a 0.9% NaCl solution for medical use. The steps involved are:
1.Calculate the Mass of NaCl Required:
2.Weigh 4.5 g of NaCl using an analytical balance.
3.Add NaCl to a volumetric flask and dissolve in distilled water to reach a final volume of 500 mL.
4.Verify the Solution: Ensure the solution is homogeneous and the desired mass percentage is achieved.
This process is standard in medical laboratories to prepare intravenous fluids.
Example 2: Determining the Concentration of Acetic Acid in Vinegar
To determine the mass percentage of acetic acid in a sample of vinegar:
- Weigh the Vinegar Sample: Suppose the sample weighs 50 g.
- Perform Titration: Titrate the sample with a standard base (e.g., NaOH) to determine the amount of acetic acid present.
- Calculate the Mass of Acetic Acid: From the titration data, calculate the mass of acetic acid in the sample.
- Calculate Mass Percentage:
This method is commonly used in food quality control to ensure product consistency.
Importance of Mass Percentage in Industrial Chemistry
Mass percentage (% m/m) is not just a classroom calculation—it plays a vital role in industrial chemistry. In industries, it is used to ensure that chemical reactions occur with the correct proportions of reactants. Miscalculations in mass percentages can lead to inefficient reactions, safety hazards, or substandard product quality.
Key industrial applications include:
- Pharmaceutical Manufacturing: Ensuring correct active ingredient ratios in tablets and syrups. For instance, a painkiller tablet might require precisely 500 mg of acetaminophen within a 1 g tablet to ensure efficacy and safety.
- Food Industry: Standardizing recipes in large-scale production, such as sugar content in beverages or acidity in vinegar. Maintaining consistent mass percentages ensures taste uniformity and compliance with food regulations.
- Cosmetic Formulation: Maintaining safe and effective concentrations of active ingredients, such as salicylic acid in acne treatments or glycerin in moisturizers.
The accuracy of these percentages directly affects product performance, consumer safety, and regulatory compliance.
Tables of Common Mass Percentages in Everyday Substances
Below is a detailed table showing mass percentages of common substances in everyday products and industrial mixtures. This helps professionals quickly reference typical values for design, quality control, or research.
| Product/Compound | Typical Mass Percentage (% m/m) | Notes and Application |
|---|---|---|
| Sodium chloride (table salt) | 95–99% | Used in food and chemical industries |
| Ethanol in hand sanitizer | 60–95% | Effective antimicrobial solution |
| Sugar in soft drinks | 8–12% | Controls sweetness and caloric content |
| Acetic acid in vinegar | 4–8% | Culinary and cleaning applications |
| Hydrogen peroxide in cleaning | 3–6% | Disinfection and bleaching |
| Calcium carbonate in toothpaste | 20–50% | Abrasive and whitening function |
| Citric acid in beverages | 0.05–0.5% | Flavor enhancement and pH adjustment |
| Sodium bicarbonate in baking | 20–30% | Leavening agent |
| Chlorine in pool water | 1–3% | Disinfection and algae control |
| Sodium hydroxide in drain cleaners | 10–20% | Strong alkaline for chemical cleaning |
This table allows chemists, lab technicians, and production engineers to quickly assess the expected composition of common mixtures and solutions.
Mass Percentage in Environmental Chemistry
Environmental scientists frequently rely on mass percentage calculations to monitor pollutants in air, water, and soil. Examples include:
- Water Analysis: Determining the concentration of dissolved salts or heavy metals, such as lead or arsenic, in drinking water. Accurate mass percentages ensure compliance with environmental standards.
- Air Pollution Monitoring: Calculating the mass percentage of particulate matter or chemical pollutants like sulfur dioxide in urban air. Regulatory agencies often set permissible mass percentage thresholds for air quality.
- Soil Contamination Studies: Measuring pesticide residues or heavy metal content in soil samples helps in risk assessment and remediation planning.
Mass percentage offers a clear, quantitative measure that enables regulatory compliance and environmental safety.
Real-World Example: Quality Control in Beverage Production
A soft drink manufacturer needs to ensure a sugar solution has exactly 10% sugar by mass. The quality control process involves:
- Sampling the produced beverage.
- Measuring total mass and mass of dissolved sugar.
- Verifying that the measured mass percentage matches the intended formulation.
If the mass percentage deviates from the target, adjustments are made either by diluting the solution or adding sugar. This ensures consistency, taste reliability, and regulatory compliance.
Real-World Example: Pharmaceutical Tablet Formulation
Consider a tablet containing 500 mg of active ingredient within a total tablet mass of 1 g:
- The mass percentage of the active ingredient is 50%.
- This precise percentage ensures the correct dosage for therapeutic effectiveness.
- Deviations in mass percentage can compromise patient safety or efficacy of the medicine.
Pharmaceutical companies employ strict analytical techniques to measure and control these percentages during manufacturing.
Factors Affecting Mass Percentage Accuracy
Several factors can influence the accuracy of mass percentage measurements:
- Moisture Content: Water absorption can artificially increase total mass, lowering the apparent mass percentage of the component.
- Impurities: Contaminants in raw materials can skew mass percentage calculations.
- Measurement Precision: Analytical balances must be calibrated to avoid errors in component mass or total mass measurements.
- Temperature and Pressure: Changes in temperature or pressure can affect the mass of volatile substances in mixtures.
By controlling these factors, laboratories and industries can ensure highly accurate mass percentage calculations.
Practical Tips for Reliable Mass Percentage Calculations
- Use Calibrated Equipment: Always use analytical balances with high precision.
- Account for All Components: Include solvents, stabilizers, or fillers in the total mass calculation.
- Repeat Measurements: Multiple measurements reduce random error.
- Standardize Procedures: Follow standardized protocols (e.g., ASTM, ISO, or USP methods).
- Document Conditions: Record temperature, pressure, and moisture content for reproducibility.