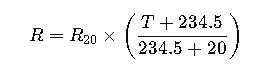Unlock the secrets of acid strength by calculating [H⁺] concentration accurately; this method governs numerous chemical reactions in industrial processes.
Explore our article detailing formulas, tables, examples, and FAQs, ensuring complete mastery of [H⁺] concentration calculations today with proven techniques.
AI-powered calculator for Calculation of [H⁺] Concentration
Example Prompts
- Calculate [H⁺] for pH = 3.5
- Determine [H⁺] when pH = 7.0
- Find [H⁺] concentration for pH = 2.0
- [H⁺] analysis at pH = 5.5
Understanding [H⁺] Concentration and pH Fundamentals
Acid-base chemistry is foundational in numerous engineering and scientific applications. Calculation of [H⁺] concentration is pivotal for quantifying the acidity of a solution, determining the reactivity and stability of chemical compounds, and regulating industrial processes.
The [H⁺] concentration is inherently related to pH, a logarithmic scale that represents the strength of an acid or base. Accurate measurements and calculations help engineers optimize reactions, enhance product quality, and ensure safety in chemical handling.
Acidic solutions contain a high concentration of hydrogen ions (H⁺), whereas basic solutions exhibit low H⁺ concentrations. The pH scale ranges from 0 to 14, with values lower than 7 being acidic and values higher than 7 basic.
Understanding these fundamentals is crucial when designing experiments, formulating products in the pharmaceutical, chemical, and food industries, and performing quality control in environmental laboratories.
Key Formulas for Calculation of [H⁺] Concentration
The primary relationship between pH and hydrogen ion concentration is defined by the formula:
This equation enables the determination of [H⁺] given a known pH value, where [H⁺] is expressed in moles per liter (M).
Similarly, the pH of a solution can be calculated if the hydrogen ion concentration is known using the inverse relationship: pH = -log [H⁺]. These logarithmic relationships highlight the sensitivity of pH measurements to changes in [H⁺] and reinforce the importance of accurate concentration calculations in research and engineering applications.
Detailed Explanation of the Variables
In the formula [H⁺] = 10-pH, every variable plays a significant role in the accuracy of the calculation:
- [H⁺]: Represents the molar concentration of hydrogen ions in the solution. This value is measured in moles per liter (M) and is crucial for understanding the acidity strength.
- pH: Represents the negative logarithm of the hydrogen ion concentration. It is a dimensionless number that describes the acidity or alkalinity of a solution on a scale from 0 to 14.
The formula establishes a direct mathematical relationship where a decrease in pH by one unit corresponds to a tenfold increase in the hydrogen ion concentration, making the function highly sensitive to small changes in pH value.
Additional Formulas for Related Calculations
Beyond the basic relationship, there are extended calculations in acid-base chemistry that are integral to the broader determination of [H⁺] concentration:
- Dissociation Equilibrium: For weak acids, the dissociation equilibrium is shown by:
HA ⇌ H⁺ + A⁻
The acid dissociation constant (Ka) is defined as:
Ka = ([H⁺][A⁻]) / [HA]where [HA] is the concentration of the undissociated acid, and [A⁻] is the concentration of its conjugate base.
- Dilution Effects: When acid solutions are diluted, the concentration [H⁺] changes according to:
C₁V₁ = C₂V₂
where C₁ and C₂ are the initial and final concentrations, while V₁ and V₂ are the corresponding volumes.
These additional formulas complement the primary calculation and are used when evaluating the behavior of acids in various reactions, including titration analysis, buffer solutions, and industrial-scale chemical production.
Extensive Tables for Calculation of [H⁺] Concentration
Below are a series of tables that elucidate different scenarios for calculation of [H⁺] concentration and associated parameters.
Table 1: Sample pH to [H⁺] Conversion
| pH Value | [H⁺] (M) | Order of Magnitude Change |
|---|---|---|
| 1.0 | 1 × 10-1 | High |
| 3.0 | 1 × 10-3 | Moderate |
| 7.0 | 1 × 10-7 | Neutral |
| 10.0 | 1 × 10-10 | Low |
Table 2: Acid Concentration and pH Interdependencies
| Acid Concentration (M) | pH | [H⁺] (Calculated) |
|---|---|---|
| 0.1 | 1.0 | 1 × 10-1 |
| 0.001 | 3.0 | 1 × 10-3 |
| 1 × 10-7 | 7.0 | 1 × 10-7 |
| 1 × 10-10 | 10.0 | 1 × 10-10 |
Real-World Applications and Detailed Examples
In many industrial and laboratory scenarios, accurate calculation of [H⁺] concentration is indispensable. Two detailed cases illustrate the practical implications of these calculations.
Case Study 1: Titration in a Pharmaceutical Laboratory
During the formulation of a new drug, a pharmaceutical laboratory performs a titration experiment to determine the concentration of an acid used as a reactant. Engineers carefully record pH values during incremental additions of a base. The experimental data reveals a gradual decrease in pH until the equivalence point is reached.
Step 1: Initially, the acid solution has a pH of 2.5. The [H⁺] is calculated as:
Step 2: As the titration progresses, careful monitoring ensures that the pH increases accordingly. At the equivalence point, the pH near 7.0 yields:
Step 3: Detailed analysis of the titration curve provides critical information about the acid’s dissociation constant (Ka). With the known [H⁺] and the observed pH change, engineers calculate Ka using the equation mentioned earlier.
This practical application validates the importance of understanding and accurately calculating [H⁺] concentration during titration, ensuring effective quality control and adherence to regulatory standards in pharmaceuticals. Moreover, such accurate analysis aids in optimizing formulations to achieve the desired therapeutic effect.
Case Study 2: Environmental Monitoring of Acid Rain
In environmental engineering, monitoring the acidity of rainwater is essential to assess its impact on aquatic life and infrastructure. Engineers collect samples from various locations and measure the pH to evaluate the [H⁺] concentration in the rainwater.
Step 1: In one region, the measured pH of the rainwater is recorded as 4.2. The hydrogen ion concentration is determined as:
Step 2: Additional samples with pH readings between 4.0 and 5.0 are similarly analyzed. The data is consolidated to create a regional acidity profile that identifies areas with higher environmental risk. Engineers use these values to calculate the average [H⁺] concentration, which is then compared with historical data to monitor trends.
Step 3: The comprehensive analysis involves creating maps and charts that depict the variation in [H⁺] concentration across different geographical areas. This informs mitigation strategies such as the introduction of neutralizing agents in particularly affected water bodies.
The environmental case underscores how the calculation of [H⁺] concentration supports decision-making processes in environmental protection. Accurate pH measurements and [H⁺] calculations enable regulators to establish environmental standards and drive policy changes for pollution control. Detailed assessments like these have been endorsed by agencies such as the U.S. Environmental Protection Agency (EPA), highlighting the practical relevance of these calculations.
Advanced Considerations in [H⁺] Concentration Calculations
Accurate calculation of [H⁺] concentration is not only about applying a simple logarithmic formula; engineers must consider additional parameters that might impact the measurement accuracy.
It is crucial to factor in temperature, ionic strength, and the presence of interfering substances in the solution. Temperature variations can alter the dissociation equilibrium, while the ionic strength of the solution might impact the activity coefficients of the ions. Calibration of pH meters and proper experimental setup are essential to minimize errors.
For instance, when correcting for temperature effects, adjustments using the Nernst equation might be necessary. Although the standard [H⁺] calculation remains as [H⁺] = 10-pH, the measured pH could be offset due to temperature-dependent behavior. Engineers sometimes calibrate instruments at the measurement temperature to ensure precision in calculated values.
- Instrument Calibration: Regular calibration with standard buffer solutions ensures that the pH meter readings are accurate.
- Interference Correction: The presence of other ions may require corrections using activity coefficients.
- Temperature Adjustment: Instruments and calculations might need adjustments based on the ambient temperature to maintain accuracy.
These advanced considerations ensure that the calculated [H⁺] concentrations are reliable, which is especially important when the results inform critical industrial, environmental, or healthcare decisions.
Using Modern Software and Tools for [H⁺] Calculations
The evolution of digital technology has greatly simplified the computation of [H⁺] concentration. Various software tools and online calculators offer engineers fast and accurate results.
Modern laboratory information management systems (LIMS) integrate pH data with automated calculations, reducing human error and increasing efficiency. These tools also support data visualization, such as plotting titration curves or mapping acid rain distribution, which aid in comprehensive analysis.
Cloud-based applications and dedicated smartphone apps are now available for field engineers. They provide real-time data processing, enabling immediate calculation of [H⁺] concentration from measured pH values. This level of accuracy and speed is crucial for industries ranging from pharmaceuticals to environmental monitoring.
Many of these platforms incorporate algorithmic corrections for temperature, ionic strengths, and other variables, ensuring that users receive reliable outputs regardless of the surrounding conditions. For further details, refer to authoritative resources such as the American Chemical Society (ACS) and the International Union of Pure and Applied Chemistry (IUPAC).
Frequently Asked Questions (FAQs)
-
What is the relationship between pH and [H⁺] concentration?
The relationship is logarithmic. [H⁺] is calculated as 10-pH, meaning a one-unit decrease in pH results in a tenfold increase in [H⁺]. -
How do I calculate the pH from [H⁺] concentration?
pH is determined using pH = -log [H⁺]. Using a scientific calculator or online tool can simplify this computation. -
Why is accurate [H⁺] measurement important in industrial processes?
Accurate measurements of [H⁺] help maintain product quality, optimize reactions, prevent corrosion, and comply with environmental and safety regulations. -
How does temperature affect the [H⁺] concentration calculation?
Temperature can influence the dissociation equilibrium and activity coefficients. Calibration and temperature corrections are necessary for precise measurements. -
Where can I find reliable resources for further learning?
You can refer to sources like the American Chemical Society (ACS) or the National Institute of Standards and Technology (NIST) for in-depth information and guidelines.
The above FAQs address common concerns related to the calculation of [H⁺] concentration. They provide concise explanations that complement the detailed analysis presented in this article.
Practical Tips for Accurate [H⁺] Concentration Determination
Achieving precise [H⁺] concentration calculations requires both theoretical understanding and practical considerations in the laboratory or field.
Ensure that your pH meter is properly calibrated and maintained. Use standardized buffer solutions for calibration and routinely check the instrument’s accuracy. Always consider environmental factors such as temperature and ionic strength during measurements.
- Regular Calibration: Calibrate instruments at the beginning and during experiments to prevent drift in measurements.
- Proper Sample Handling: Avoid contamination and ensure that samples are stored properly before analysis.
- Environmental Controls: Maintain a consistent temperature and robust methodology to reduce variability.
- Data Logging: Use digital tools to record and analyze pH data continuously for improved reliability.
Employing these practical tips in combination with the theoretical foundations ensures robust and accurate measurement of [H⁺] concentration, fundamental to quality control and experimental reproducibility.
Integrating [H⁺] Concentration Calculations into Industrial Processes
In industries such as pharmaceuticals, food processing, and environmental engineering, the calculation of [H⁺] concentration is deeply integrated into overall operational procedures.
For example, quality control laboratories continuously monitor the acidity of solutions to ensure that product specifications are met. Automated systems measure pH in real time and feed these values into integrated software that calculates the [H⁺] concentration instantly.
Furthermore, in large-scale manufacturing, feedback loops using these measurements can adjust process parameters dynamically. This allows for enhanced process control, reduced waste, and optimized chemical reactions. These systems seamlessly integrate with supervisory control and data acquisition (SCADA) systems, ensuring accurate monitoring and timely intervention.
Case Examples in Industry
Engineers in the food industry employ [H⁺] concentration calculations during the fermentation process to control the growth of beneficial microorganisms while inhibiting harmful bacteria.
An example is the production of yogurt. During fermentation, the pH decreases gradually as lactic acid is produced. Monitoring [H⁺] concentration enables manufacturers to determine the optimal point for terminating the fermentation process, ensuring product consistency and safety.
Similarly, wastewater treatment facilities utilize [H⁺] concentration calculations to adjust the neutralization process. By measuring the pH and calculating the hydrogen ion concentration, operators add precise quantities of neutralizing agents to achieve the desired pH for safe discharge. Such applications not only protect the environment but also comply with stringent regulatory standards.
Future Trends and Innovations
The field of acid-base chemistry, and specifically the calculation of [H⁺] concentration, continues to evolve.
Advancements in sensor technology, data analytics, and machine learning are expected to further refine precision and accuracy. Emerging biosensors and nanotechnology-based devices promise faster response times and miniaturization, enabling real-time monitoring in even the most challenging environments.
Furthermore, integration with the Industrial Internet of Things (IIoT) is on the horizon. This integration will usher in smarter monitoring systems with predictive maintenance capabilities, improved safety protocols, and enhanced energy efficiency. Industry 4.0 applications are already demonstrating the potential to significantly improve process control by using continuous digital feedback loops.
Conclusion and Recommendations for Practitioners
Accurate calculation of [H⁺] concentration is a cornerstone in chemical engineering, environmental science, and industrial processes.
Engineers and scientists must combine robust theoretical frameworks with sound practical methodologies to ensure reliability in pH measurements and subsequent [H⁺] determinations. This article has detailed the critical formulas, provided explanatory tables, showcased real-world applications, and answered common FAQs to offer a comprehensive resource.
We recommend practitioners focus on regular instrument calibration, consider environmental influencing factors, and incorporate modern digital tools to enhance measurement precision. Adopting these best practices will improve process control, product quality, and research outcomes.
Additional Resources and Authoritative Links
For further reading and in-depth guidelines, please visit the following authoritative resources:
- American Chemical Society (ACS)
- National Institute of Standards and Technology (NIST)
- International Union of Pure and Applied Chemistry (IUPAC)
- U.S. Environmental Protection Agency (EPA)
These external links provide additional verified information and standards that ensure you remain informed about the latest innovations and regulatory guidelines in the calculation of [H⁺] concentration.
Summary
The calculation of [H⁺] concentration is not only a fundamental chemical equation but also a critical tool used across industries to monitor and control processes.
From enhancing drug formulation in pharmaceutical laboratories to safeguarding the environment through careful titration of acid rain, the practical applications of this method are far-reaching. With clear formulas, detailed explanations of variables, extensive tables, and real-world case studies, this resource aims to empower you with knowledge and tools imperative for your engineering and scientific endeavors.
Embrace these insights, consistently verify your measurements, and utilize modern digital tools to achieve reliability and precision. The benefits of mastering the calculation of [H⁺] concentration extend to improved safety, robust research, and optimized industrial operations. Continue exploring and applying these principles for success in your professional field.